Downloads
Keywords:
End-to-End Validation of Clinical Trial Management Systems Using Flexible Methodologies
Authors
Abstract
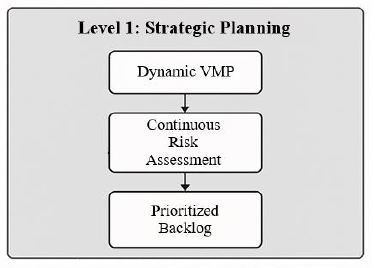
The article is devoted to the investigation and development of a comprehensive model for end-to-end (E2E) validation of clinical trial management systems (CTMS) using Agile methodologies. The relevance of the work is driven by the need to accelerate the market entry of new medicinal products while regulatory requirements for data integrity are simultaneously tightening. The novelty lies in proposing an integrated framework that combines Agile principles, a risk-based approach, and continuous compliance verification into a single process for GxP environments. The study describes the limitations of traditional waterfall validation models and examines modern approaches to software validation in the pharmaceutical industry. Special attention is paid to developing a dynamic validation master plan and adapting CI/CD pipelines for regulated environments. The work sets the objective of developing a conceptual model that enables improved operational efficiency and data quality while maintaining compliance with regulatory standards. To achieve this, the methods used include systematic analysis of the scientific literature, comparative analysis, and conceptual modeling. The conclusion describes the practical significance of the proposed model for pharmaceutical companies and suggests directions for further research. The materials presented in the article will be of interest to validation specialists, quality managers, IT leaders, and data management specialists in the life sciences domain.
Article Details
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

